When I introduced NOTCH3 as the major actor in CADASIL, I described its expression in blood vessels in the brain. The protein receives a signal from a neighboring cell before being cleaved and migrating to the receiving cell’s nucleus. We’re going to dig into that a little deeper.
Pathways and Ligands
First, we need to arm ourselves with a few bits of vocabulary. A biological pathway means a series of interactions between molecules that lead to the production of new materials, or change in the shape or behavior of a cell.
A signaling pathway, or signal transduction is typically a pathway that begins with a molecule from outside the cell interacting with another on the surface of the cell. This results in a cascade of events that change the cell’s behavior. Signal pathways often begin outside the cell membrane and end in the nucleus of the cell. The final product is often represented as more of a new protein, or the guided destruction of an older one.
A ligand is just a substance. Usually a protein or some other molecule, that binds to another for some functional purpose.
Notch
The NOTCH signal messaging pathway is called “highly conserved” in all animals. This just means it appeared quite early in evolution. We know it’s incredibly useful for all animal life because we see it everywhere in Nature, from the simplest fly to the most sophisticated apes like ourselves. Mammals have 4 different versions of NOTCH, NOTCH1-4, each with its own unique purpose.
But qhy is NOTCH even called “NOTCH”? When I began to study the NOTCH signaling pathway, I saw that it interacted with other molecules, ligands, referred to as Serrate and Jagged, and I assumed that it had to do with the shape of the molecules themselves – biologists tend to have fun when naming the proteins or molecules they discover. A jagged molecule could latch onto a notched one quite easily!
However, I learned that NOTCH was discovered first in the fruit fly, Drosophila Melanogaster, the result of cross breeding experiments by Thomas Hunt Morgan and John S. Dexter back in the early 1900’s. Flies were described as having a “notch at the end of the wings”, and from there it stuck, becoming the name of the gene when it was cloned several decades later. Here is the drawing of the notched wings from Dexter’s paper in the American Naturalist from 1914:
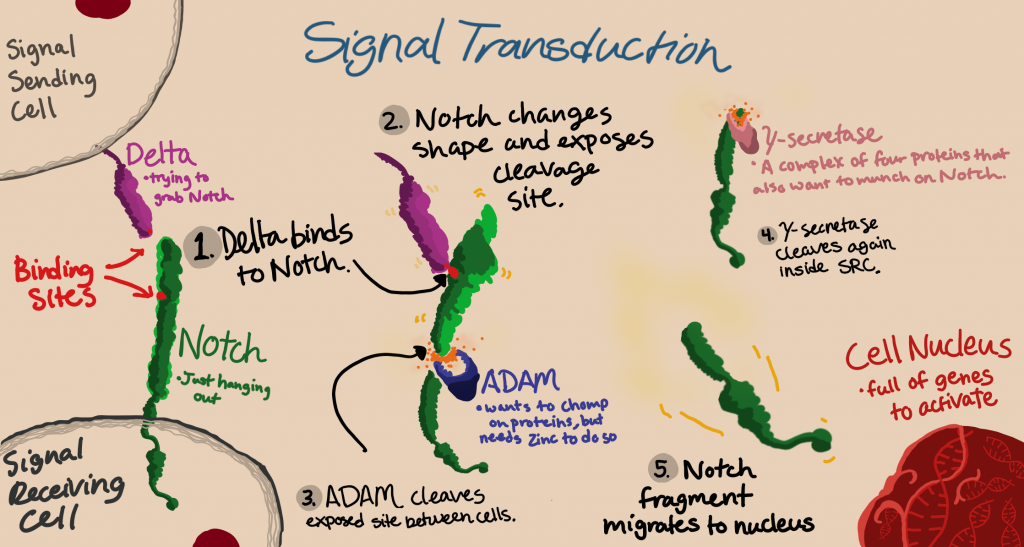
In the NOTCH pathway, the ligand (called Delta or Jagged in mammals) is exposed on the surface of a signal-sending cell adjacent to a signal-receiving cell. A portion of the NOTCH protein protrudes from each. The two proteins physically interact, latching onto each other like a handshake, and a metalloprotease called ADAM breaks the NOTCH protein. ADAM acts on the region that just-barely pokes out of the cell membrane, called the Negative Regulatory Region, or NRR. It’s called a negative regulatory region because when NOTCH isn’t latched onto Delta, NOTCH coils up differently, and the cleavage site for ADAM is physically hidden.
NOTCH is quickly cleaved again by another protease called γ-secretase, which allows the intracellular domain of NOTCH to detach completely and begin its migration to the nucleus. This cleavage event happens quite close to the cell membrane as well, but on the inside of the cell. The larger, extracellular portion of NOTCH remains bound to Delta/Jagged.
Notch3 and CASASIL
It’s difficult to immediately associate these tiny cell-to-cell interactions, orchestrated by even smaller movements of miniscule molecules, with disease – but the importance of NOTCH3’s role in CADASIL becomes defined here. It’s also where the CADASIL Project-sponsored research at the Starita Lab becomes relevant. Mutations in NOTCH3 can result in the accumulation of the longer segment between the signal sending and signal receiving cells. We’re investigating not only which mutations may result in that accumulation, but also how it forms, and how it may cease correct cell-to-cell communications.
Both dementia and stroke are major symptoms of CADASIL, either resulting from that intercellular accumulation, or improper gene regulation from the fragment that migrates deeper into the cell to change gene regulation. In my next post, we’ll look at a how different types of mutations in NOTCH3 may lead to different levels or symptoms of disease progression.